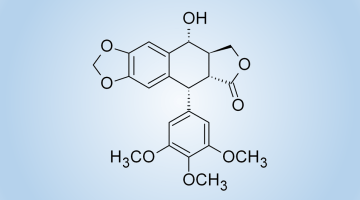
The oxidation of melatonin with singlet molecular oxygen (O2 (1Δg)) may produce cyclic 3-hydroxymelatonin 1. We made several melatonin analogs having carbamate substituent instead of the methoxy group at 5 position of the indole ring. These compounds behave analogously to melatonin with respect to singlet oxygen treatment and produce the corresponding cyclic 3-hydroxymelatonin analogs. The compounds obtained possess the 2,3,8,8a-tetrahydropyrrolo[2,3-b]indole heterocyclic system which is a structural motif characteristic of alkaloids physostigmine 2 and related compounds that are potent acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors used in the Alzheimer’s disease treatment.
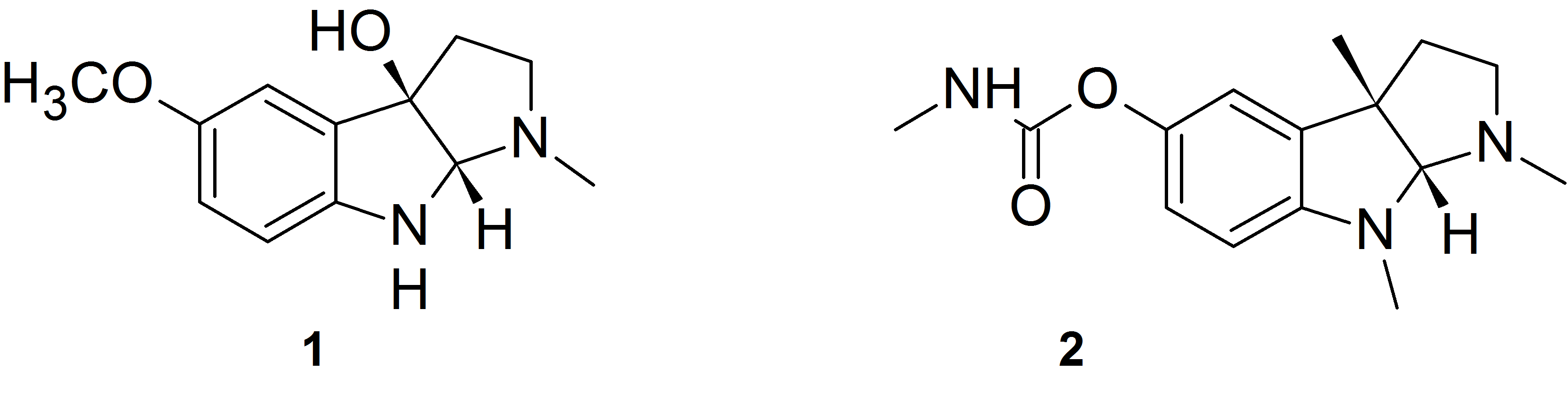
We measured the inhibition activity of the obtained several compounds against AChE and BChE from human erythrocytes and serum.
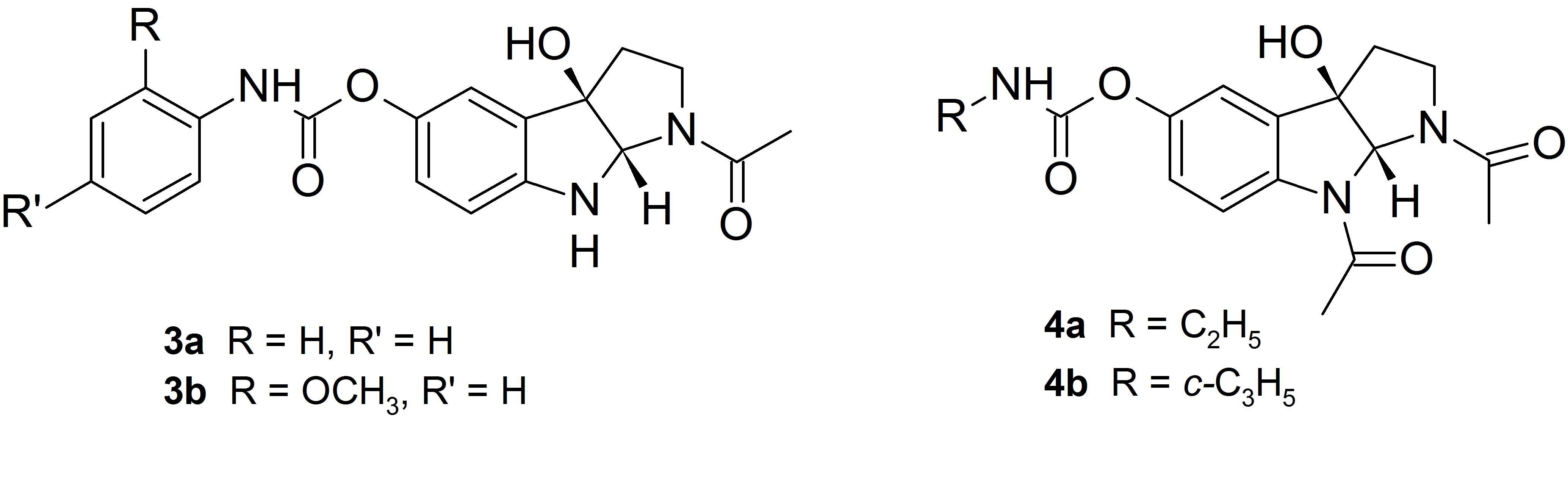
The best inhibitors of AChE proved phenyl and metoxyphenylcarbamate derivatives 3a and 3b, while the alkyl carbamate analogs 4a and 4b show selectivity toward inhibition of butyrylcholinesterase.
Recently, our studies on new cholinesterase inhibitors expanded to include a group called hybrid drugs – compounds combining in their structure two fragments, a known drug and its copy or two different drugs.
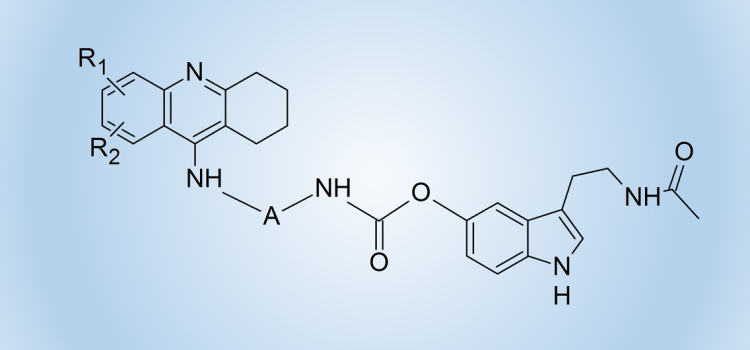
We used this idea and the method of substitution of melatonin and its oxidation products via a carbamate bond involving the phenolic oxygen atom to obtain heterodimers containing a known drug for the treatment of Alzheimer’s disease – tacrine and melatonin (compounds of structure 5) or its oxidation product (compounds of structure 6).
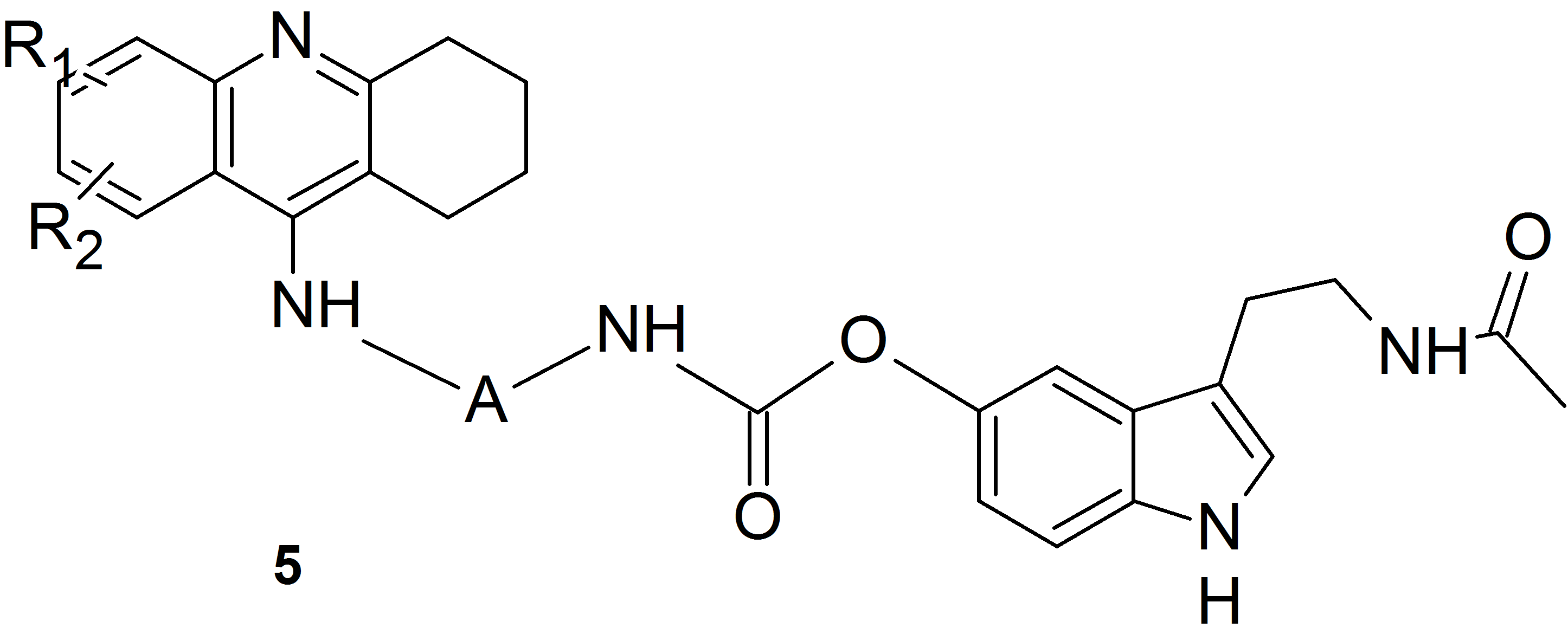
We obtained a series of derivatives with different substituents in the tacrine ring and various number of methylene units in the linker between the amine and the carbamate groups.
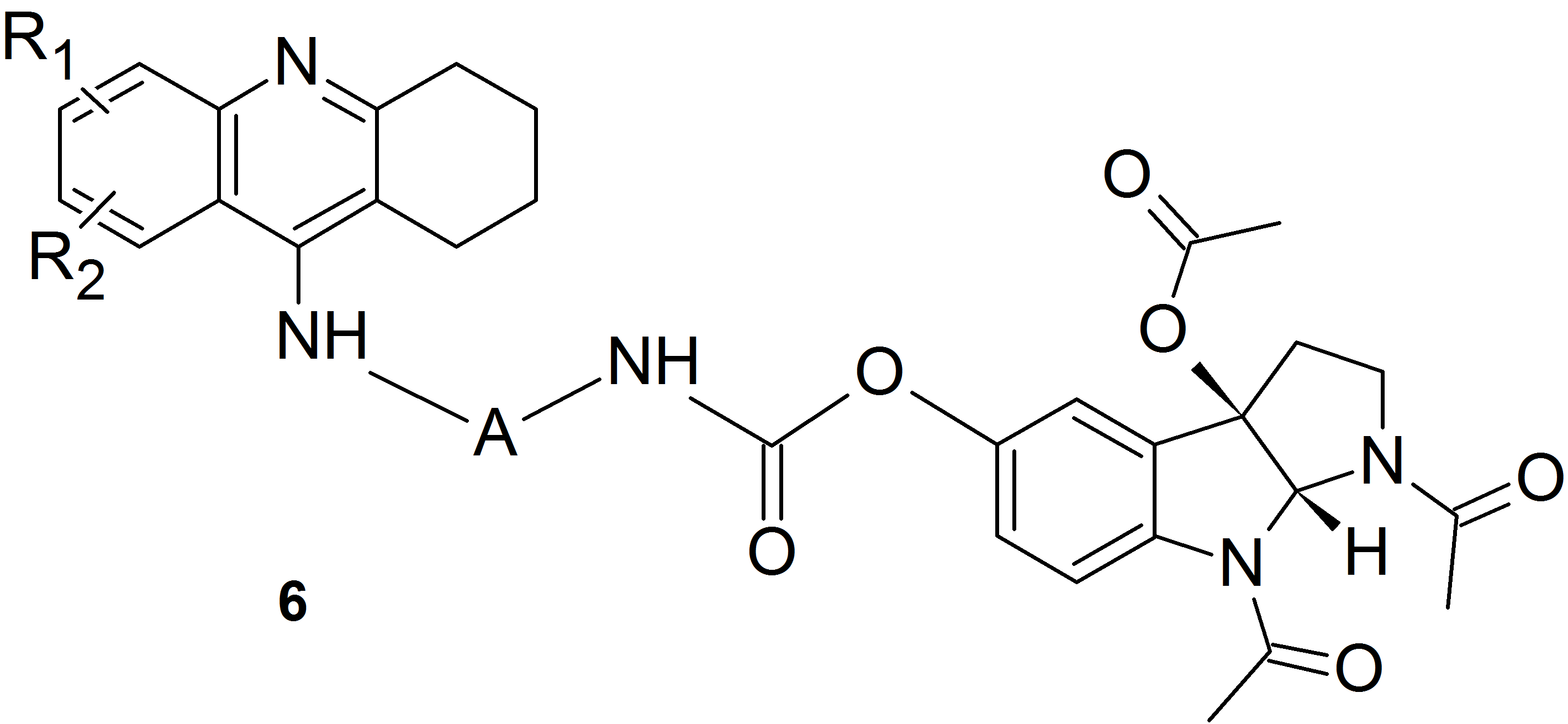
These heterodimers exhibit a much higher cholinesterase inhibitory activity than previously described derivatives containing melatonin or its oxidation products units or compounds in which melatonin and tetrahydroacridine units are connected via a linker containing an amide bond.
The new hybrid cholinesterases inhibitors are protected by patent and can be used in relief and/or treatment of the neurodegenerative disorders, among them the Alzheimer’s disease.
Siwicka A., Molęda Z., Wojtasiewicz K., Zawadzka A., Maurin J. K., Panasiewicz M., Pacuszka T., Czarnocki Z. “The oxidation products of melatonin derivatives exhibit acetylcholinesterase and butyrylcholinesterase inhibitory activity” J. Pineal Res., 45, 40-49 (2008)
Molęda Z., Wojtasiewicz K., Panasiewicz M., Czarnocki Z. “Selective inhibition of butyrylcholinesterase by singlet oxygen generated melatonin derivatives” J. Pineal Res., 49, 55-59 (2010)
Zawadzka A., Łozińska I., Molęda Z., Panasiewicz M., Czarnocki Z. “Highly selective inhibition of butyrylcholinesterase by a novel melatonin–tacrine heterodimers” J. Pineal Res., 54, 435-441 (2013)
Zawadzka A., Czarnocki Z., Łozińska I., Molęda Z., Panasiewicz M. “Novel Hybrid Cholinesterase Inhibitors” US Patent No.: 8,841,453 B2; Sep. 23, 2014.
Zawadzka A., Czarnocki Z., Łozińska I., Molęda Z., Panasiewicz M. “Novel Hybrid Cholinesterase Inhibitors” EP No.: 2 714 658 B1; Apr. 29, 2015.
Zawadzka A., Czarnocki Z., Łozińska I., Molęda Z., Panasiewicz M. “New cholinesterase inhibitors of hybrid structure” PL Patent No.: 219106 B1; Mar. 31, 2015.