Atropisomerism is a type of stereochemistry being a consequence of the hindered rotational barrier of suitably substituted biaryls and analogous compounds. The role of atropisomerism in both chemistry and biology has already been well-documented. Many biologically active compounds exist in the form of pure atropisomers and this phenomenon has important implications for medicinal chemistry.
During our collaboration with Internal Security Agency we synthesized many oligoaryl-substituted heterocyclic compounds using the Suzuki-Miyaura coupling reaction.
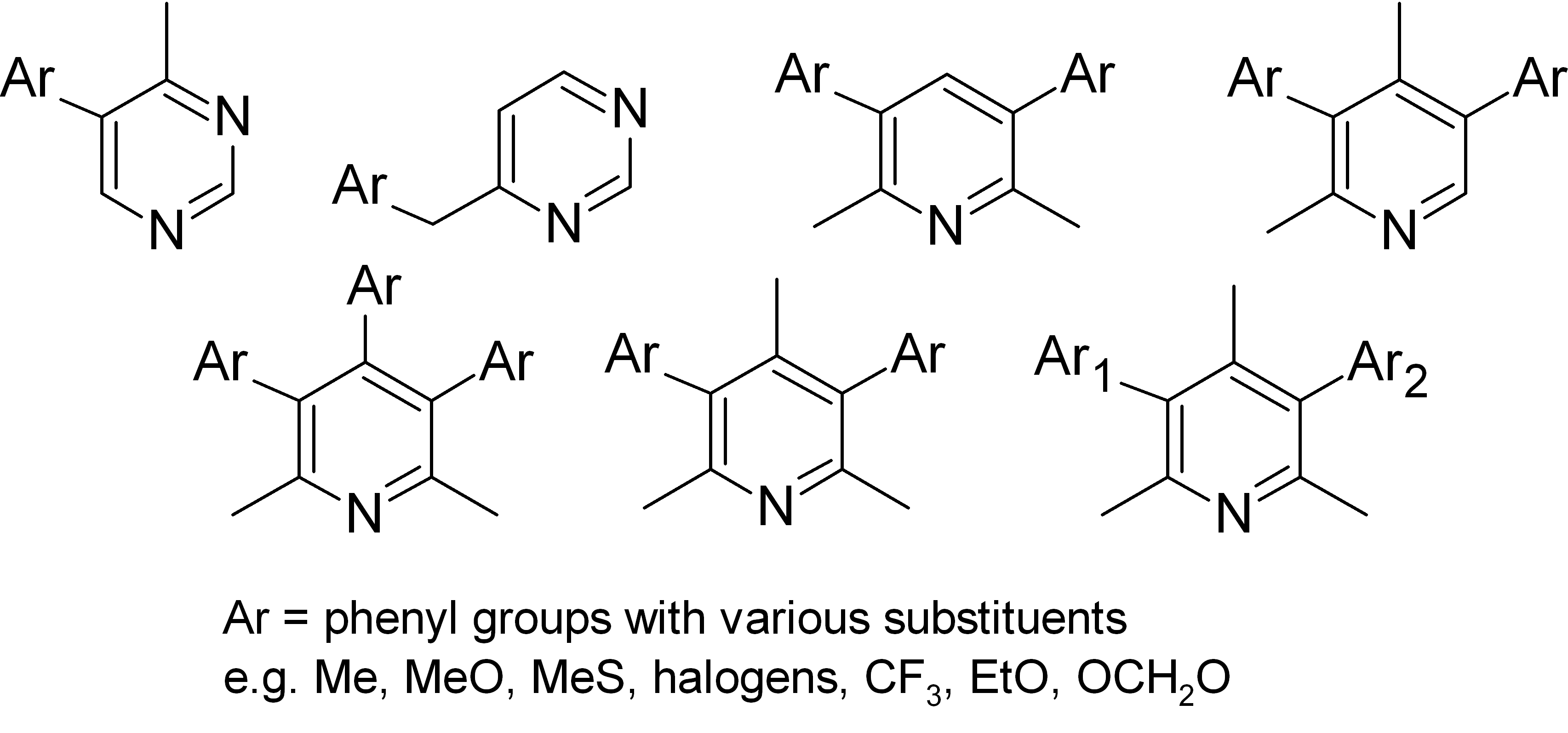
In some cases we observed the presence of stable stereoisomers derived from the presence of chiral axis.
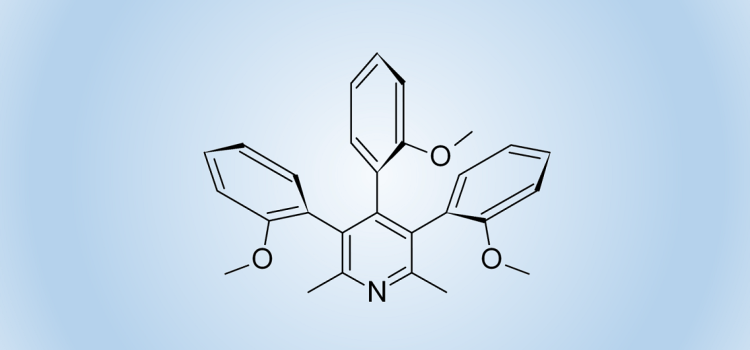
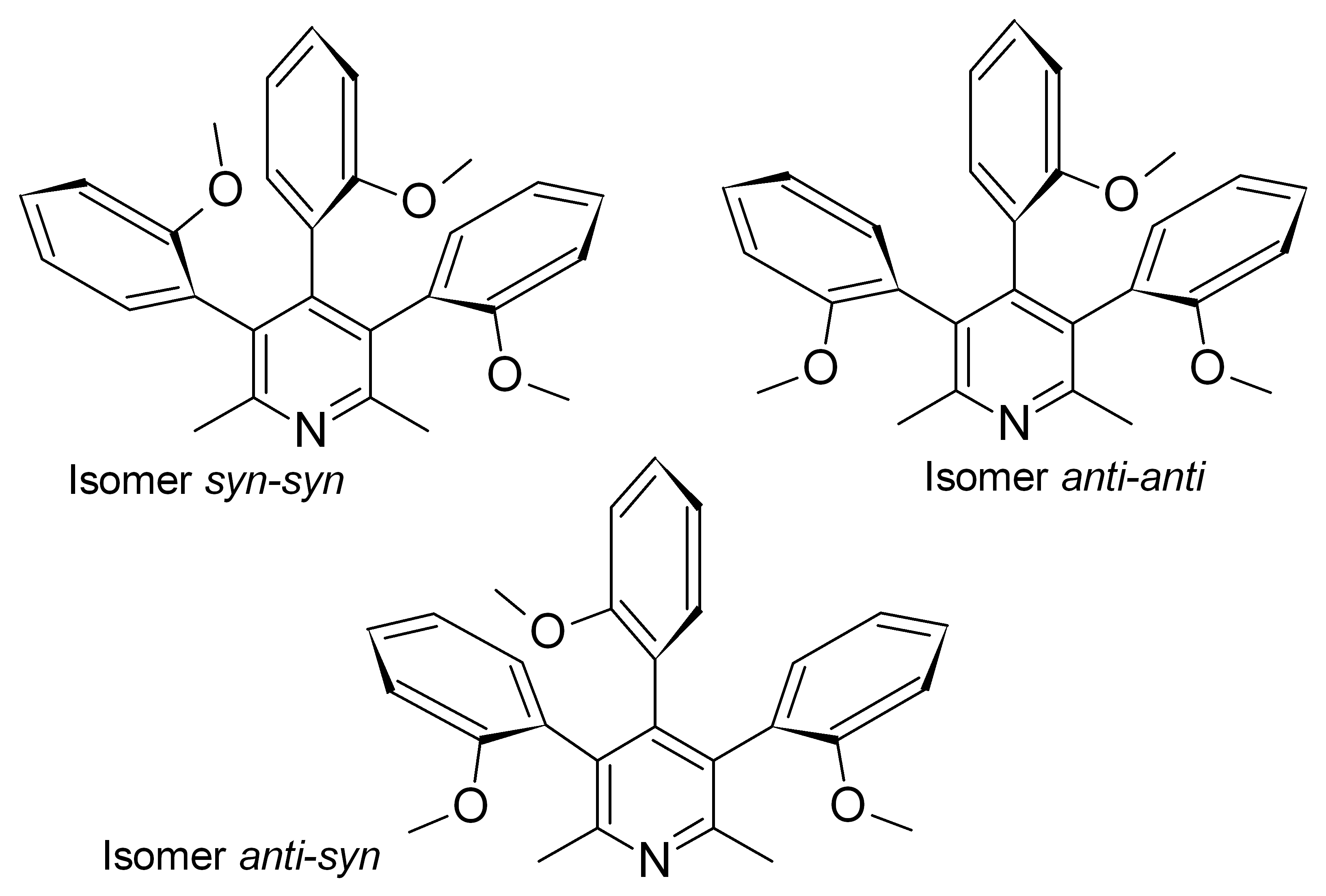
For example, we found that the Suzuki reaction of tribromo-2,6-lutidine with 2-methoxyphenylboronic acid gave 3,4,5-tri-(2-methoxyphenyl)-2,6-lutidine in the form of a mixture of three stable at room temperature diastereomers. Each of them was isolated and fully characterized, including X-ray structure determination. Chiral isomer anti-syn, being a racemic mixture, was separated into individual enantiomers. Their absolute stereochemistry was assigned with the computational calculation of the CD spectra and was also finally confirmed by X-ray analysis. Thermal interconversion of atropisomers was studied with time-dependent NMR and dynamic HPLC accompanied by quantum chemical methods.
Similar phenomena were observed also in the case of 4-amino-2,6-lutidine derivatives.
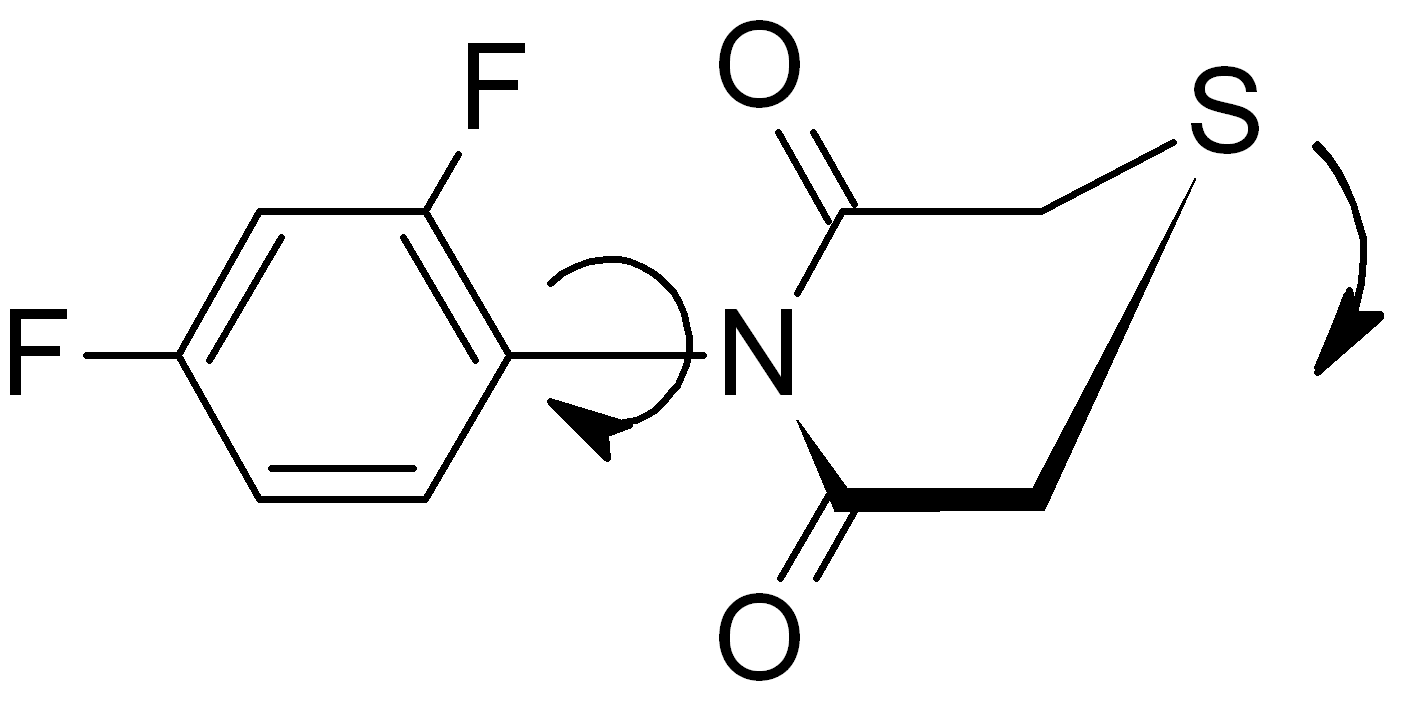
We also prepared a series of previously unknown N-aryl-substituted thiomorpholine-3,5-diones. In the case of (2-phenyl)-substituted derivatives, stable at room temperature diastereomers were detected in 1H NMR. The dynamic stereochemistry of selected compounds was studied with variable-temperature 1H NMR and the mechanism of diastereomers interconversion was proposed in the basis of quantum chemical calculations.
Błachut D., Wojtasiewicz K., Krawczyk K., Maurin J.K., Szawkało J., Czarnocki Z., „Identification and synthesis of by-products found in 4-methylthioamphetamine (4-MTA) produced by the Leuckart method” Forensic Sci. Int. 216, 108-120 (2012)
Roszkowski P., Błachut D., Maurin J.K., Woźnica M., Frelek J., Pluciński F., Czarnocki Z., „Atropisomerism in 3,4,5-Tri-(2-methoxyphenyl)-2,6-lutidine” European Journal of Organic Chemistry 7867-7871 (2013)
Szawkało J., Maurin J.K., Pluciński F., Czarnocki Z., „Synthesis and dynamic stereochemistry of 4-aryl-thiomorpholine-3,5-dione derivatives” J Mol Struct 1079, 383-390 (2015)